Ajuda'ns a fer que la transparència alimentària sigui la norma!
Com a organització sense ànim de lucre, depenem de les vostres donacions per continuar informant els consumidors de tot el món sobre tot allò què mengen.
La revolució alimentària comença amb tu!
Berocca - bayer - 144g (30 x 4.8g)
Berocca - bayer - 144g (30 x 4.8g)
Aquesta pàgina del producte no està completa. Podeu ajudar a completar-la editant-la i afegint-hi més dades a partir de les fotos ja disponibles, o fent-ne més amb l'aplicació de androide o iPhone / iPad. Gràcies!
×
Codi de barres: 8470001716835 (EAN / EAN-13)
Nom comú: Berocca
Quantitat: 144g (30 x 4.8g)
Empaquetament: Plàstic, en:container
Marques: Bayer
Categories: Suplements dietètics
Etiquetes, certificacions, premis:
Punt verd
Codi de traçabilitat: CN 171683.5
Enllaç a la pàgina del producte en el lloc oficial del productor: https://www.berocca.com
Països on es va vendre: Espanya
Matching with your preferences
Salut
Ingredients
-
36 ingredients
Castellà: Acidulantes: ácido cítrico, carbonato ácido de sodio; ácido L-ascórbico, sulfato magnésico; agente de carga: isomalt; carbonato cálcico; carbonato magnésico; agente de carga: sorbitol; aroma de naranja; acidulante: carbonato de sodio; nicotinamida; cloruro sódico; colorante: beta-caroteno; citrato de zinc; colorante: rojo de remolacha; D-pantotenato cálcico; edulcorante: aspartamo; riboflavina-5'-fosfato sódico; edulcorante: acesulfamo potásico; tiamina clorhidrato; agente de carga: manitol; piridoxina clorhidrato; cianocobalamina; antiespumante: polisorbato 60; ácido pteroilmonoglutámico; D-biotina.
Processament d'aliments
-
Aliments ultra processats
Elements que indiquen que el producte està al grup 4 - Aliments i begudes ultraprocessats:
- Additiu: E101 - Riboflavina
- Additiu: E160a - Carotè
- Additiu: E162 - Betanina
- Additiu: E420 - Sorbitol
- Additiu: E421 - Mannitol
- Additiu: E435 - Monoestearat de sorbitan polioxietilenat
- Additiu: E950 - Acesulfam K
- Additiu: E951 - Aspartam
- Additiu: E953 - Isomalt
- Ingredient: Antiespumant
- Ingredient: Incrementador de volum
- Ingredient: Color
- Ingredient: Aromes
- Ingredient: Edulcorant
Els productes alimentaris es classifiquen en 4 grups segons el seu grau de processament:
- Aliments no processats o mínimament processats
- Ingredients culinaris processats
- Aliments processats
- Aliments ultra processats
La determinació del grup es fa en funció de la categoria del producte i dels ingredients que conté.
Additius
-
E101 - Riboflavina
Riboflavin: Riboflavin, also known as vitamin B2, is a vitamin found in food and used as a dietary supplement. Food sources include eggs, green vegetables, milk and other dairy product, meat, mushrooms, and almonds. Some countries require its addition to grains. As a supplement it is used to prevent and treat riboflavin deficiency and prevent migraines. It may be given by mouth or injection.It is nearly always well tolerated. Normal doses are safe during pregnancy. Riboflavin is in the vitamin B group. It is required by the body for cellular respiration.Riboflavin was discovered in 1920, isolated in 1933, and first made in 1935. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Riboflavin is available as a generic medication and over the counter. In the United States a month of supplements costs less than 25 USD.Origen: Wikipedia (Anglès)
-
E101i - Riboflavina
Riboflavin: Riboflavin, also known as vitamin B2, is a vitamin found in food and used as a dietary supplement. Food sources include eggs, green vegetables, milk and other dairy product, meat, mushrooms, and almonds. Some countries require its addition to grains. As a supplement it is used to prevent and treat riboflavin deficiency and prevent migraines. It may be given by mouth or injection.It is nearly always well tolerated. Normal doses are safe during pregnancy. Riboflavin is in the vitamin B group. It is required by the body for cellular respiration.Riboflavin was discovered in 1920, isolated in 1933, and first made in 1935. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Riboflavin is available as a generic medication and over the counter. In the United States a month of supplements costs less than 25 USD.Origen: Wikipedia (Anglès)
-
E160a - Carotè
Carotene: The term carotene -also carotin, from the Latin carota, "carrot"- is used for many related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but in general cannot be made by animals -with the exception of some aphids and spider mites which acquired the synthesizing genes from fungi-. Carotenes are photosynthetic pigments important for photosynthesis. Carotenes contain no oxygen atoms. They absorb ultraviolet, violet, and blue light and scatter orange or red light, and -in low concentrations- yellow light. Carotenes are responsible for the orange colour of the carrot, for which this class of chemicals is named, and for the colours of many other fruits, vegetables and fungi -for example, sweet potatoes, chanterelle and orange cantaloupe melon-. Carotenes are also responsible for the orange -but not all of the yellow- colours in dry foliage. They also -in lower concentrations- impart the yellow coloration to milk-fat and butter. Omnivorous animal species which are relatively poor converters of coloured dietary carotenoids to colourless retinoids have yellowed-coloured body fat, as a result of the carotenoid retention from the vegetable portion of their diet. The typical yellow-coloured fat of humans and chickens is a result of fat storage of carotenes from their diets. Carotenes contribute to photosynthesis by transmitting the light energy they absorb to chlorophyll. They also protect plant tissues by helping to absorb the energy from singlet oxygen, an excited form of the oxygen molecule O2 which is formed during photosynthesis. β-Carotene is composed of two retinyl groups, and is broken down in the mucosa of the human small intestine by β-carotene 15‚15'-monooxygenase to retinal, a form of vitamin A. β-Carotene can be stored in the liver and body fat and converted to retinal as needed, thus making it a form of vitamin A for humans and some other mammals. The carotenes α-carotene and γ-carotene, due to their single retinyl group -β-ionone ring-, also have some vitamin A activity -though less than β-carotene-, as does the xanthophyll carotenoid β-cryptoxanthin. All other carotenoids, including lycopene, have no beta-ring and thus no vitamin A activity -although they may have antioxidant activity and thus biological activity in other ways-. Animal species differ greatly in their ability to convert retinyl -beta-ionone- containing carotenoids to retinals. Carnivores in general are poor converters of dietary ionone-containing carotenoids. Pure carnivores such as ferrets lack β-carotene 15‚15'-monooxygenase and cannot convert any carotenoids to retinals at all -resulting in carotenes not being a form of vitamin A for this species-; while cats can convert a trace of β-carotene to retinol, although the amount is totally insufficient for meeting their daily retinol needs.Origen: Wikipedia (Anglès)
-
E160ai
Beta-Carotene: β-Carotene is an organic, strongly colored red-orange pigment abundant in plants and fruits. It is a member of the carotenes, which are terpenoids -isoprenoids-, synthesized biochemically from eight isoprene units and thus having 40 carbons. Among the carotenes, β-carotene is distinguished by having beta-rings at both ends of the molecule. β-Carotene is biosynthesized from geranylgeranyl pyrophosphate.β-Carotene is the most common form of carotene in plants. When used as a food coloring, it has the E number E160a. The structure was deduced by Karrer et al. in 1930. In nature, β-carotene is a precursor -inactive form- to vitamin A via the action of beta-carotene 15‚15'-monooxygenase.Isolation of β-carotene from fruits abundant in carotenoids is commonly done using column chromatography. It can also be extracted from the beta-carotene rich algae, Dunaliella salina. The separation of β-carotene from the mixture of other carotenoids is based on the polarity of a compound. β-Carotene is a non-polar compound, so it is separated with a non-polar solvent such as hexane. Being highly conjugated, it is deeply colored, and as a hydrocarbon lacking functional groups, it is very lipophilic.Origen: Wikipedia (Anglès)
-
E162 - Betanina
Betanin: Betanin, or Beetroot Red, is a red glycosidic food dye obtained from beets; its aglycone, obtained by hydrolyzing away the glucose molecule, is betanidin. As a food additive, its E number is E162. The color of betanin depends on pH; between four and five it is bright bluish-red, becoming blue-violet as the pH increases. Once the pH reaches alkaline levels betanin degrades by hydrolysis, resulting in a yellow-brown color. Betanin is a betalain pigment, together with isobetanin, probetanin, and neobetanin. Other pigments contained in beet are indicaxanthin and vulgaxanthins.Origen: Wikipedia (Anglès)
-
E330 - Acid citric
Citric acid: Citric acid is a weak organic acid that has the chemical formula C6H8O7. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than a million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring and chelating agent.A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate ion is written as C6H5O3−7 or C3H5O-COO-3−3.Origen: Wikipedia (Anglès)
-
E420 - Sorbitol
Sorbitol: Sorbitol --, less commonly known as glucitol --, is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the aldehyde group to a hydroxyl group. Most sorbitol is made from corn syrup, but it is also found in nature, for example in apples, pears, peaches, and prunes. It is converted to fructose by sorbitol-6-phosphate 2-dehydrogenase. Sorbitol is an isomer of mannitol, another sugar alcohol; the two differ only in the orientation of the hydroxyl group on carbon 2. While similar, the two sugar alcohols have very different sources in nature, melting points, and uses.Origen: Wikipedia (Anglès)
-
E421 - Mannitol
Mannitol: Mannitol is a type of sugar alcohol which is also used as a medication. As a sugar, it is often used as a sweetener in diabetic food, as it is poorly absorbed from the intestines. As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to lower increased intracranial pressure. Medically, it is given by injection. Effects typically begin within 15 minutes and last up to 8 hours.Common side effects from medical use include electrolyte problems and dehydration. Other serious side effects may include worsening heart failure and kidney problems. It is unclear if use is safe in pregnancy. Mannitol is in the osmotic diuretic family of medications and works by pulling fluid from the brain and eyes.The discovery of mannitol is attributed to Joseph Louis Proust in 1806. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The wholesale cost in the developing world is about US$1.12 to 5.80 a dose. In the United States, a course of treatment costs $25 to 50. It was originally made from the flowering ash and called manna due to its supposed resemblance to the Biblical food. Mannitol is on the World Anti-Doping Agency's banned drug list due to concerns that it may mask other drugs.Origen: Wikipedia (Anglès)
-
E500 - Carbonats de sodi
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Origen: Wikipedia (Anglès)
-
E500i - Carbonat de sodi
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Origen: Wikipedia (Anglès)
-
E500ii - Bicarbonat de sodi
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Origen: Wikipedia (Anglès)
-
E950 - Acesulfam K
Acesulfame potassium: Acesulfame potassium - AY-see-SUL-faym-, also known as acesulfame K -K is the symbol for potassium- or Ace K, is a calorie-free sugar substitute -artificial sweetener- often marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number -additive code- E950. It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG -now Nutrinova-. In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1‚2,3-oxathiazine-4-3H--one 2‚2-dioxide. It is a white crystalline powder with molecular formula C4H4KNO4S and a molecular weight of 201.24 g/mol.Origen: Wikipedia (Anglès)
-
E951 - Aspartam
Aspartame: Aspartame -APM- is an artificial non-saccharide sweetener used as a sugar substitute in some foods and beverages. In the European Union, it is codified as E951. Aspartame is a methyl ester of the aspartic acid/phenylalanine dipeptide. A panel of experts set up by the European Food Safety Authority concluded in 2013 that aspartame is safe for human consumption at current levels of exposure. As of 2018, evidence does not support a long-term benefit for weight loss or in diabetes. Because its breakdown products include phenylalanine, people with the genetic condition phenylketonuria -PKU- must be aware of this as an additional source.It was first sold under the brand name NutraSweet. It was first made in 1965, and the patent expired in 1992. It was initially approved for use in food products by the U.S. Food and Drug Administration -FDA- in 1981. The safety of aspartame has been the subject of several political and medical controversies, United States congressional hearings, and Internet hoaxes.Origen: Wikipedia (Anglès)
-
E953 - Isomalt
Isomalt: Isomalt is a sugar substitute, a type of sugar alcohol used primarily for its sugar-like physical properties. It has little to no impact on blood sugar levels, and does not stimulate the release of insulin. It also does not promote tooth decay, i.e. is tooth-friendly. Its energy value is 2 kcal/g, half that of sugars. However, like most sugar alcohols, it carries a risk of gastric distress, including flatulence and diarrhea, when consumed in large quantities -above about 20-30 g per day-. Isomalt may prove upsetting to the intestinal tract because it is incompletely absorbed in the small intestine, and when polyols pass into the large intestine, they can cause osmotically induced diarrhea and stimulate the gut flora, causing flatulence. As with other dietary fibers, regular consumption of isomalt can lead to desensitisation, decreasing the risk of intestinal upset. Isomalt can be blended with high-intensity sweeteners such as sucralose, giving a mixture that has the same sweetness as sugar. Isomalt is an equimolar mixture of two mutually diastereomeric disaccharides, each composed of two sugars: glucose and mannitol -α-D-glucopyranosido-1‚6-mannitol- and also glucose and sorbitol -α-D-glucopyranosido-1‚6-sorbitol-. Complete hydrolysis of isomalt yields glucose -50%-, sorbitol -25%-, and mannitol -25%-. It is an odorless, white, crystalline substance containing about 5% water of crystallisation. Isomalt has a minimal cooling effect -positive heat of solution-, lower than many other sugar alcohols, in particular, xylitol and erythritol. Isomalt is manufactured in a two-stage process in which sucrose is first transformed into isomaltulose, a reducing disaccharide -6-O-α-D-glucopyranosido-D-fructose-. The isomaltulose is then hydrogenated, using a Raney nickel catalyst. The final product — isomalt — is an equimolar composition of 6-O-α-D-glucopyranosido-D-sorbitol -1‚6-GPS- and 1-O-α-D-glucopyranosido-D-mannitol-dihydrate -1‚1-GPM-dihydrate-. Isomalt has been approved for use in the United States since 1990. It is also permitted for use in Australia, New Zealand, Canada, Mexico, Iran, the European Union, and other countries. Isomalt is widely used for the production of sugar-free candy, especially hard-boiled candy, because it resists crystallisation much better than the standard combinations of sucrose and corn syrup. It is used in sugar sculpture for the same reason.Origen: Wikipedia (Anglès)
Anàlisi dels ingredients
-
Pot contenir oli de palma
Ingredients que poden contenir oli de palma: Beta-carotè
-
Es desconeix si és vegà
Ingredients no reconeguts: es:sulfato-magnesico, es:carbonato-magnesico, Clorur de sodi, es:citrato-de-zinc, es:riboflavina-5-fosfato-sodico, es:tiamina-clorhidrato, es:piridoxina-clorhidrato, en:Cyanocobalamin, Àcid fòlicAlguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
-
Es desconeix si és vegetarià
Ingredients no reconeguts: es:sulfato-magnesico, es:carbonato-magnesico, Clorur de sodi, es:citrato-de-zinc, es:riboflavina-5-fosfato-sodico, es:tiamina-clorhidrato, es:piridoxina-clorhidrato, en:Cyanocobalamin, Àcid fòlicAlguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
-
Detalls de l'anàlisi dels ingredients
Necessitem la teva ajuda!
Alguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
es: Acidulantes (ácido cítrico), carbonato ácido de sodio, ácido L-ascórbico, sulfato magnésico, agente de carga (isomalt), carbonato cálcico, carbonato magnésico, agente de carga (sorbitol), aroma de naranja, acidulante (carbonato de sodio), nicotinamida, cloruro sódico, colorante (beta-caroteno), citrato de zinc, colorante (rojo de remolacha), D-pantotenato cálcico, edulcorante (aspartamo), riboflavina-5'-fosfato sódico, edulcorante (acesulfamo potásico), tiamina clorhidrato, agente de carga (manitol), piridoxina clorhidrato, cianocobalamina, antiespumante (polisorbato 60), ácido pteroilmonoglutámico, D-biotina- Acidulantes -> en:acid - percent_min: 3.84615384615385 - percent_max: 100
- ácido cítrico -> en:e330 - vegan: yes - vegetarian: yes - percent_min: 3.84615384615385 - percent_max: 100
- carbonato ácido de sodio -> en:e500ii - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 50
- ácido L-ascórbico -> en:e300 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 33.3333333333333
- sulfato magnésico -> es:sulfato-magnesico - percent_min: 0 - percent_max: 25
- agente de carga -> en:bulking-agent - percent_min: 0 - percent_max: 20
- isomalt -> en:e953 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 20
- carbonato cálcico -> en:e170i - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 16.6666666666667
- carbonato magnésico -> es:carbonato-magnesico - percent_min: 0 - percent_max: 14.2857142857143
- agente de carga -> en:bulking-agent - percent_min: 0 - percent_max: 12.5
- sorbitol -> en:e420 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 12.5
- aroma de naranja -> en:orange-flavouring - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 5
- acidulante -> en:acid - percent_min: 0 - percent_max: 5
- carbonato de sodio -> en:e500i - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 5
- nicotinamida -> en:e375 - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 5
- cloruro sódico -> en:sodium-chloride - percent_min: 0 - percent_max: 5
- colorante -> en:colour - percent_min: 0 - percent_max: 5
- beta-caroteno -> en:e160ai - vegan: maybe - vegetarian: maybe - from_palm_oil: maybe - percent_min: 0 - percent_max: 5
- citrato de zinc -> es:citrato-de-zinc - percent_min: 0 - percent_max: 5
- colorante -> en:colour - percent_min: 0 - percent_max: 5
- rojo de remolacha -> en:e162 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 5
- D-pantotenato cálcico -> en:d-pantothenate-calcium - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 5
- edulcorante -> en:sweetener - percent_min: 0 - percent_max: 5
- aspartamo -> en:e951 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 5
- riboflavina-5'-fosfato sódico -> es:riboflavina-5-fosfato-sodico - percent_min: 0 - percent_max: 5
- edulcorante -> en:sweetener - percent_min: 0 - percent_max: 5
- acesulfamo potásico -> en:e950 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 5
- tiamina clorhidrato -> es:tiamina-clorhidrato - percent_min: 0 - percent_max: 5
- agente de carga -> en:bulking-agent - percent_min: 0 - percent_max: 4.76190476190476
- manitol -> en:e421 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 4.76190476190476
- piridoxina clorhidrato -> es:piridoxina-clorhidrato - percent_min: 0 - percent_max: 4.54545454545455
- cianocobalamina -> en:cyanocobalamin - percent_min: 0 - percent_max: 4.34782608695652
- antiespumante -> en:anti-foaming-agent - percent_min: 0 - percent_max: 4.16666666666667
- polisorbato 60 -> en:e435 - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 4.16666666666667
- ácido pteroilmonoglutámico -> en:folic-acid - percent_min: 0 - percent_max: 4
- D-biotina -> en:biotin - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 3.84615384615385
Nutrició
-
Falten dades per calcular la Nutri-Score
Falten dades nutricionals
⚠ ️S'han d'especificar les dades nutricionals del producte per calcular el Nutri-Score.Podries afegir la informació necessària per calcular el Nutri-Score? Afegir dades nutricionals
-
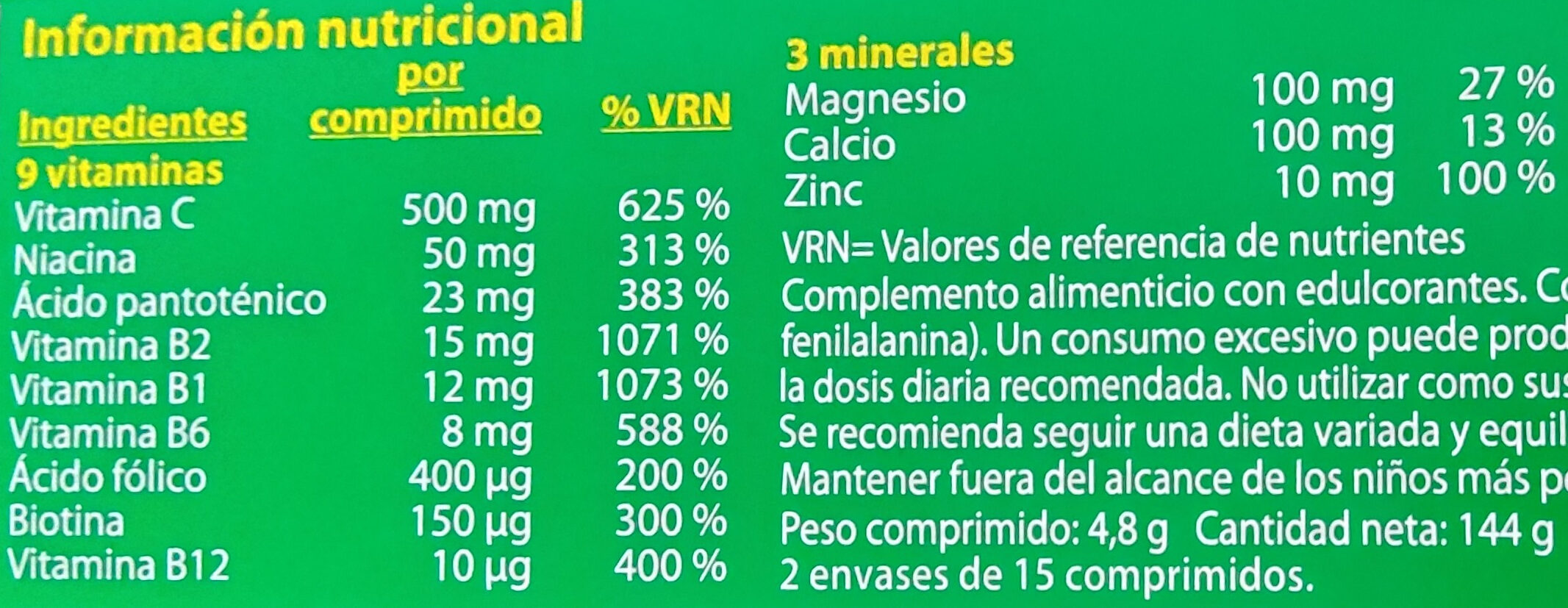
Informació nutricional
Informació nutricional Com es ven
per 100 g/100 mlCom es ven
per porció (4.8 g)Comparat amb: Suplements dietètics Greix ? ? Àcid gras saturat ? ? Hidrats de carboni ? ? Sucre ? ? Fiber ? ? Proteïna ? ? Sal comuna ? ? Vitamina C ? 500 mg Vitamin B1 (Thiamin) ? 12 mg Vitamin B2 (Riboflavin) ? 15 mg Vitamin B3 ? 50 mg Vitamina B6 ? 8 mg Vitamin B9 (Folic acid) ? 400 µg Vitamin B12 (cobalamin) ? 10 µg Biotina ? 150 µg Àcid pantotènic ? 23 mg Calci ? 100 mg Magnesi ? 100 mg Zinc ? 10 mg Fruits‚ vegetables‚ nuts and rapeseed‚ walnut and olive oils (estimate from ingredients list analysis) 0 % 0 %
Entorn
-
Puntuació ecològica no calculada - Impacte ambiental desconegut
No hem pogut calcular l'Eco-Score d'aquest producte perquè li falten algunes dades, podríeu ajudar-nos a completar-lo?Podries afegir una categoria del producte més precisa perquè puguem calcular l'Eco-Score? Afegir una categoria
Empaquetament
-
Embalatge d'impacte mitjà
-
Peces d'embalatge
(Plàstic)
-
Materials d'embalatge
Material % Pes de l'embalatge Pes de l'embalatge per 100 g de producte Plàstic
-
Transport
-
Orígens dels ingredients
Falta informació sobre l'origen dels ingredients
⚠ ️ L'origen dels ingredients d'aquest producte no està indicat.
Si estan indicats a l'embalatge, podeu modificar la fitxa del producte i afegir-los.
Si sou el fabricant d'aquest producte, podeu enviar-nos la informació amb la nostra plataforma gratuïta per a productors.Add the origins of ingredients for this product Add the origins of ingredients for this product
Report a problem
-
Incomplete or incorrect information?
Category, labels, ingredients, allergens, nutritional information, photos etc.
If the information does not match the information on the packaging, please complete or correct it. Open Food Facts is a collaborative database, and every contribution is useful for all.
Fonts de dades
Producte afegit per kiliweb
Última modificació de la pàgina del producte per duhowpi.
La pàgina del producte, també editada per bh88, musarana, packbot, thaialagata, yuka.ZjY0eU02VTlpK0lUbzhNNTNSZjV4dkJKekx5bFVGaTdCZGRKSUE9PQ.