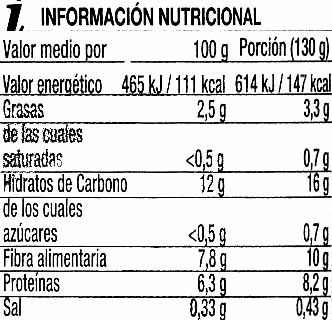
Garbanzos cocidos en conserva - SuperSol - 560 g (neto), 400 g (escurrido), 580 ml
Aquesta pàgina del producte no està completa. Podeu ajudar a completar-la editant-la i afegint-hi més dades a partir de les fotos ja disponibles, o fent-ne més amb l'aplicació de androide o iPhone / iPad. Gràcies!
×
Codi de barres: 8430803037088 (EAN / EAN-13)
Nom comú: Garbanzos cocidos en conserva. Categoría primera
Quantitat: 560 g (neto), 400 g (escurrido), 580 ml
Empaquetament: es:Bote de vidrio
Marques: SuperSol
Categories: Aliments i begudes amb base vegetal, Aliments amb base vegetal, Llegums i derivats, Aliments enllaunats, Llegums, Llavors i grans, Aliments enllaunats amb base vegetal, Llavors de llegum, Llegums secs, Cigrons, Llegums en conserva, en:Canned chickpeas
Etiquetes, certificacions, premis: Vegetarià, Vegà
Llocs de fabricació o processament: [DISTRIBUIDOR], Calahorra, La Rioja, España
Codi de traçabilitat: FABRICANTE Y ENVASADOR:, DESCONOCIDO, DISTRIBUIDOR:, COMPRE Y COMPARE S.A. (CONSERVAS CELORRIO)
Botigues: SuperSol
Països on es va vendre: Espanya