Ajuda'ns a fer que la transparència alimentària sigui la norma!
Com a organització sense ànim de lucre, depenem de les vostres donacions per continuar informant els consumidors de tot el món sobre tot allò què mengen.
La revolució alimentària comença amb tu!
Mon gâteau minute mug cake moelleux chocolat noisettes ANCEL - Dr.Oetker - 67 g
Mon gâteau minute mug cake moelleux chocolat noisettes ANCEL - Dr.Oetker - 67 g
Aquesta pàgina del producte no està completa. Podeu ajudar a completar-la editant-la i afegint-hi més dades a partir de les fotos ja disponibles, o fent-ne més amb l'aplicació de androide o iPhone / iPad. Gràcies!
×
Codi de barres: 3027030008209 (EAN / EAN-13)
Quantitat: 67 g
Empaquetament: Plàstic, en:Bag
Marques: Dr.Oetker, Ancel, Dr. Oetker
Categories: Snacks, Aperitius dolços, Galetes i pastissos, Pastís, Ajudants de cuina, en:Dessert mixes, en:Pastry helpers, en:Baking Mixes, Pastissos de xocolata, en:Cake mixes
Etiquetes, certificacions, premis:
Punt verd
Botigues: Carrefour Market, Magasins U
Matching with your preferences
Salut
Ingredients
-
24 ingredients
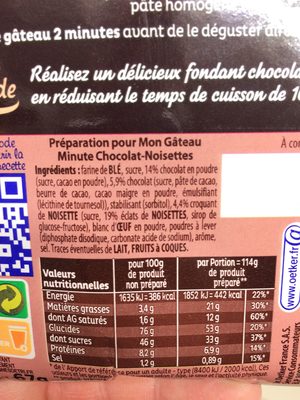
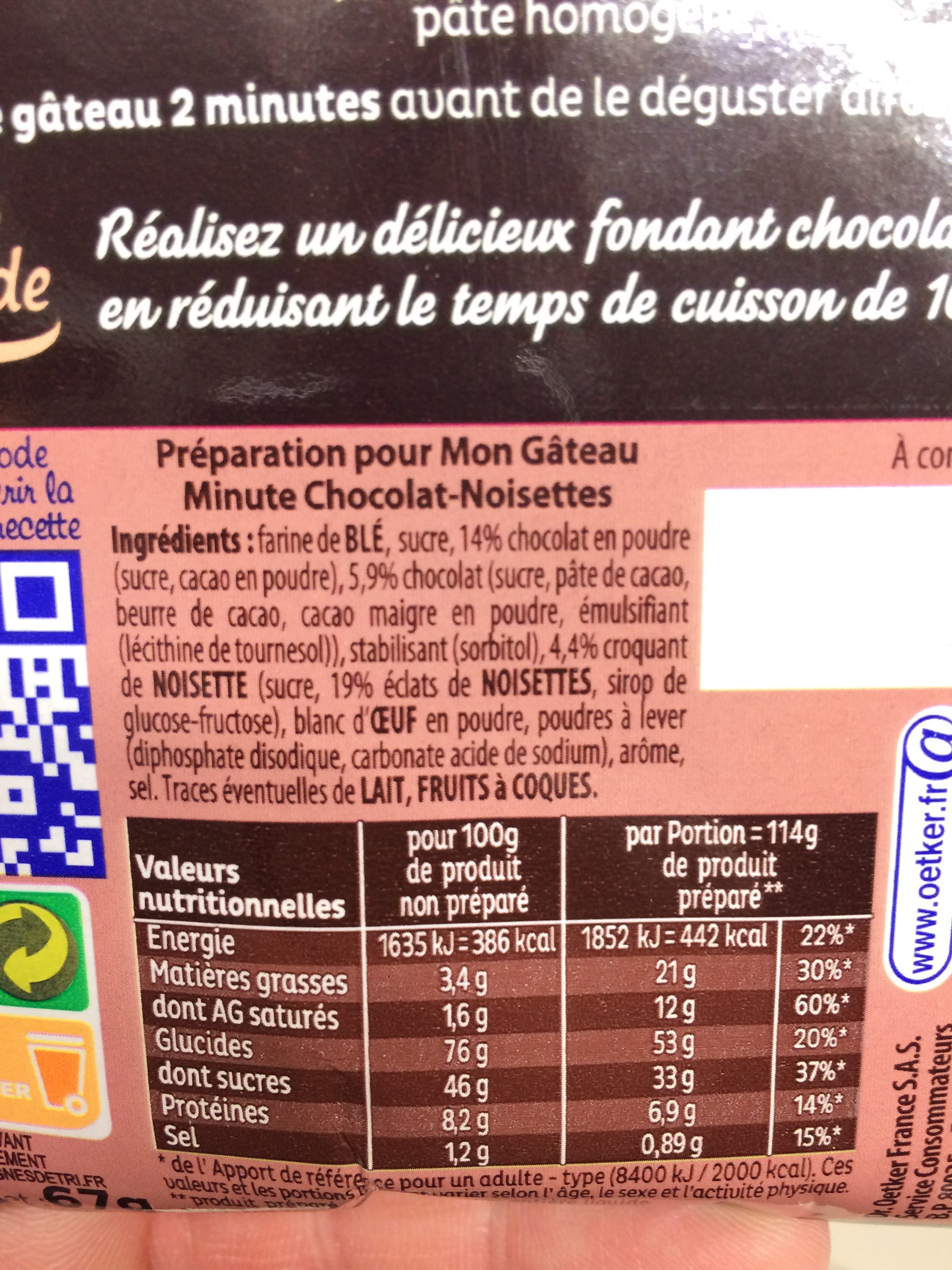
: Farine de BLÉ, sucre, 14 % chocolat en poudre (sucre, cacao en poudre), 5,9 % chocolat (sucre, pâte de cacao, beurre de cacao, cacao maigre en poudre, émulsifiant (lécithine de tournesol)), stabilisant (sorbitol), 4,4 % croquant de NOISETTE (sucre, 19 % éclats de NOISETTES, sirop de glucose-fructose), blanc d'OEUF en poudre, poudres à lever (diphosphate disodique, carbonate acide de sodium), arôme, sel.Al·lèrgens: en:Eggs, en:Gluten, en:NutsRastres: en:Milk, en:Nuts
Processament d'aliments
-
Aliments ultra processats
Elements que indiquen que el producte està al grup 4 - Aliments i begudes ultraprocessats:
- Additiu: E322 - Lecitines
- Additiu: E420 - Sorbitol
- Additiu: E450 - Difosfat
- Ingredient: Emulsionant
- Ingredient: Aromes
- Ingredient: Glucosa
Els productes alimentaris es classifiquen en 4 grups segons el seu grau de processament:
- Aliments no processats o mínimament processats
- Ingredients culinaris processats
- Aliments processats
- Aliments ultra processats
La determinació del grup es fa en funció de la categoria del producte i dels ingredients que conté.
Additius
-
E322 - Lecitines
Lecithin: Lecithin -UK: , US: , from the Greek lekithos, "egg yolk"- is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues, which are amphiphilic – they attract both water and fatty substances -and so are both hydrophilic and lipophilic-, and are used for smoothing food textures, dissolving powders -emulsifying-, homogenizing liquid mixtures, and repelling sticking materials.Lecithins are mixtures of glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acid.Lecithin was first isolated in 1845 by the French chemist and pharmacist Theodore Gobley. In 1850, he named the phosphatidylcholine lécithine. Gobley originally isolated lecithin from egg yolk—λέκιθος lekithos is "egg yolk" in Ancient Greek—and established the complete chemical formula of phosphatidylcholine in 1874; in between, he had demonstrated the presence of lecithin in a variety of biological matters, including venous blood, in human lungs, bile, human brain tissue, fish eggs, fish roe, and chicken and sheep brain. Lecithin can easily be extracted chemically using solvents such as hexane, ethanol, acetone, petroleum ether, benzene, etc., or extraction can be done mechanically. It is usually available from sources such as soybeans, eggs, milk, marine sources, rapeseed, cottonseed, and sunflower. It has low solubility in water, but is an excellent emulsifier. In aqueous solution, its phospholipids can form either liposomes, bilayer sheets, micelles, or lamellar structures, depending on hydration and temperature. This results in a type of surfactant that usually is classified as amphipathic. Lecithin is sold as a food additive and dietary supplement. In cooking, it is sometimes used as an emulsifier and to prevent sticking, for example in nonstick cooking spray.Origen: Wikipedia (Anglès)
-
E322i - Lecitina
Lecithin: Lecithin -UK: , US: , from the Greek lekithos, "egg yolk"- is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues, which are amphiphilic – they attract both water and fatty substances -and so are both hydrophilic and lipophilic-, and are used for smoothing food textures, dissolving powders -emulsifying-, homogenizing liquid mixtures, and repelling sticking materials.Lecithins are mixtures of glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acid.Lecithin was first isolated in 1845 by the French chemist and pharmacist Theodore Gobley. In 1850, he named the phosphatidylcholine lécithine. Gobley originally isolated lecithin from egg yolk—λέκιθος lekithos is "egg yolk" in Ancient Greek—and established the complete chemical formula of phosphatidylcholine in 1874; in between, he had demonstrated the presence of lecithin in a variety of biological matters, including venous blood, in human lungs, bile, human brain tissue, fish eggs, fish roe, and chicken and sheep brain. Lecithin can easily be extracted chemically using solvents such as hexane, ethanol, acetone, petroleum ether, benzene, etc., or extraction can be done mechanically. It is usually available from sources such as soybeans, eggs, milk, marine sources, rapeseed, cottonseed, and sunflower. It has low solubility in water, but is an excellent emulsifier. In aqueous solution, its phospholipids can form either liposomes, bilayer sheets, micelles, or lamellar structures, depending on hydration and temperature. This results in a type of surfactant that usually is classified as amphipathic. Lecithin is sold as a food additive and dietary supplement. In cooking, it is sometimes used as an emulsifier and to prevent sticking, for example in nonstick cooking spray.Origen: Wikipedia (Anglès)
-
E420 - Sorbitol
Sorbitol: Sorbitol --, less commonly known as glucitol --, is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the aldehyde group to a hydroxyl group. Most sorbitol is made from corn syrup, but it is also found in nature, for example in apples, pears, peaches, and prunes. It is converted to fructose by sorbitol-6-phosphate 2-dehydrogenase. Sorbitol is an isomer of mannitol, another sugar alcohol; the two differ only in the orientation of the hydroxyl group on carbon 2. While similar, the two sugar alcohols have very different sources in nature, melting points, and uses.Origen: Wikipedia (Anglès)
-
E500 - Carbonats de sodi
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Origen: Wikipedia (Anglès)
-
E500ii - Bicarbonat de sodi
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Origen: Wikipedia (Anglès)
Anàlisi dels ingredients
-
No conté oli de palma
No s'han detectat ingredients que continguin oli de palma
Ingredients no reconeguts: fr:croquant-de-noisetteAlguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
-
No és vegà
Ingredients no vegans: Clara d'ou en polsAlguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
-
Es desconeix si és vegetarià
Ingredients no reconeguts: fr:croquant-de-noisetteAlguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
-
Detalls de l'anàlisi dels ingredients
Necessitem la teva ajuda!
Alguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
: Farine de BLÉ, sucre, chocolat en poudre 14% (sucre, cacao en poudre), chocolat 5.9% (sucre, pâte de cacao, beurre de cacao, cacao maigre en poudre, émulsifiant (lécithine de tournesol)), stabilisant (sorbitol), croquant de NOISETTE 4.4% (sucre, éclats de NOISETTES 0.836%, sirop de glucose-fructose), blanc d'OEUF en poudre, poudres à lever (diphosphate disodique, carbonate acide de sodium), arôme, sel- Farine de BLÉ -> en:wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410 - percent_min: 16.55 - percent_max: 57.3
- sucre -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 14 - percent_max: 35.65
- chocolat en poudre -> en:chocolate-powder - vegan: maybe - vegetarian: yes - ciqual_food_code: 18101 - percent_min: 14 - percent: 14 - percent_max: 14
- sucre -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 7 - percent_max: 14
- cacao en poudre -> en:cocoa-powder - vegan: yes - vegetarian: yes - ciqual_food_code: 18100 - percent_min: 0 - percent_max: 7
- chocolat -> en:chocolate - vegan: maybe - vegetarian: yes - percent_min: 5.9 - percent: 5.9 - percent_max: 5.9
- sucre -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 1.18 - percent_max: 5.9
- pâte de cacao -> en:cocoa-paste - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 16030 - percent_min: 0 - percent_max: 2.95
- beurre de cacao -> en:cocoa-butter - vegan: yes - vegetarian: yes - ciqual_food_code: 16030 - percent_min: 0 - percent_max: 1.96666666666667
- cacao maigre en poudre -> en:fat-reduced-cocoa-powder - vegan: yes - vegetarian: yes - ciqual_food_code: 18100 - percent_min: 0 - percent_max: 1.475
- émulsifiant -> en:emulsifier - percent_min: 0 - percent_max: 1.18
- lécithine de tournesol -> en:sunflower-lecithin - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 1.18
- stabilisant -> en:stabiliser - percent_min: 4.4 - percent_max: 5.9
- sorbitol -> en:e420 - vegan: yes - vegetarian: yes - percent_min: 4.4 - percent_max: 5.9
- croquant de NOISETTE -> fr:croquant-de-noisette - percent_min: 4.4 - percent: 4.4 - percent_max: 4.4
- sucre -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 2.728 - percent_max: 3.564
- éclats de NOISETTES -> fr:eclats-de-noisettes - vegan: yes - vegetarian: yes - ciqual_food_code: 15004 - percent_min: 0.836 - percent: 0.836 - percent_max: 0.836
- sirop de glucose-fructose -> en:glucose-fructose-syrup - vegan: yes - vegetarian: yes - ciqual_food_code: 31077 - percent_min: 0 - percent_max: 0.836
- blanc d'OEUF en poudre -> en:powdered-egg-white - vegan: no - vegetarian: yes - ciqual_food_code: 22004 - percent_min: 0 - percent_max: 4.4
- poudres à lever -> en:raising-agent - percent_min: 0 - percent_max: 4.4
- diphosphate disodique -> en:e450i - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 4.4
- carbonate acide de sodium -> en:e500ii - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 2.2
- arôme -> en:flavouring - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 4.4
- sel -> en:salt - vegan: yes - vegetarian: yes - ciqual_food_code: 11058 - percent_min: 0 - percent_max: 4.4
Nutrició
-
Falten dades per calcular la Nutri-Score
Falten dades nutricionals
⚠ ️S'han d'especificar les dades nutricionals del producte per calcular el Nutri-Score.Podries afegir la informació necessària per calcular el Nutri-Score? Afegir dades nutricionals
-
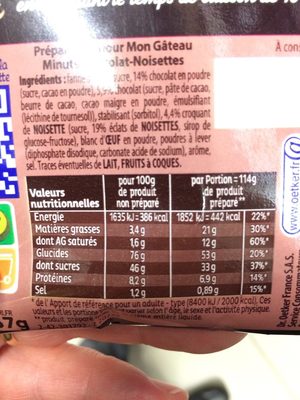
Informació nutricional
Informació nutricional Com es ven
per porcióComparat amb: en:Cake mixes Energia 1.615 kj
(386 kcal)Greix 3,4 g Àcid gras saturat 1,6 g Hidrats de carboni 76 g Sucre 46 g Fiber ? Proteïna 8,2 g Sal comuna 1,2 g Fruits‚ vegetables‚ nuts and rapeseed‚ walnut and olive oils (estimate from ingredients list analysis) 0,836 %
Entorn
-
Eco-puntuació D - Impacte ambiental alt
El Eco-Score és una puntuació experimental que resumeix els impactes ambientals dels productes alimentaris.→ L'Eco-Score es va desenvolupar inicialment a França i s'està ampliant per a altres països europeus. La fórmula Eco-Score està subjecta a canvis, ja que es millora periòdicament per fer-la més precisa i més adequada per a cada país.Anàlisi del cicle de vida
-
Impacte mitjà dels productes de la mateixa categoria: C (Score: 47/100)
Categoria: Chocolate cake
Categoria: Chocolate cake
- Puntuació ambiental PEF ( petjada ambiental de l'aliment ): 0.60 (com més baixa sigui la puntuació, menor serà l'impacte)
- incloent l'impacte sobre el canvi climàtic: 7.88 kg CO₂ eq/kg del producte
Etapa Impacte Agricultura
48.2 %Processament
45.1 %Empaquetament
3.2 %Transport
2.7 %Distribució
0.8 %Consum
0.0 %
Bonificacions i punts negatius
-
Falta informació sobre l'origen dels ingredients
Punts negatius: -5
⚠ ️ L'origen dels ingredients d'aquest producte no està indicat.
Si estan indicats a l'embalatge, podeu modificar la fitxa del producte i afegir-los.
Si sou el fabricant d'aquest producte, podeu enviar-nos la informació amb la nostra plataforma gratuïta per a productors.
-
Embalatge d'impacte mitjà
Punts negatius: -10
Forma Material Reciclatge Impacte Bag Plàstic Alt
Eco-Score per a aquest producte
-
Impacte per a aquest producte: D (Score: 32/100)
Producte: Mon gâteau minute mug cake moelleux chocolat noisettes ANCEL - Dr.Oetker - 67 g
Puntuació de l'anàlisi del cicle de vida: 47
Suma de bonificacions i punts negatius: -15
Puntuació final: 32/100
-
Petjada de carboni
-
Equivalent a conduir 4.1 km en un cotxe de gasolina
788 g de CO² per cada 100 g de producte
La xifra d'emissions de carboni prové de la base de dades Agribalyse d'ADEME, per a la categoria: Chocolate cake (Font: Base de dades ADEME Agribalyse)
Etapa Impacte Agricultura
30.6 %Processament
63.3 %Empaquetament
3.7 %Transport
2.2 %Distribució
0.2 %Consum
0.0 %
Empaquetament
-
Embalatge d'impacte mitjà
-
Peces d'embalatge
Bag (Plàstic)
-
Materials d'embalatge
Material % Pes de l'embalatge Pes de l'embalatge per 100 g de producte Plàstic
-
Transport
-
Orígens dels ingredients
Falta informació sobre l'origen dels ingredients
⚠ ️ L'origen dels ingredients d'aquest producte no està indicat.
Si estan indicats a l'embalatge, podeu modificar la fitxa del producte i afegir-los.
Si sou el fabricant d'aquest producte, podeu enviar-nos la informació amb la nostra plataforma gratuïta per a productors.Add the origins of ingredients for this product Add the origins of ingredients for this product
Report a problem
-
Incomplete or incorrect information?
Category, labels, ingredients, allergens, nutritional information, photos etc.
If the information does not match the information on the packaging, please complete or correct it. Open Food Facts is a collaborative database, and every contribution is useful for all.
Fonts de dades
Producte afegit per date-limite-app
Última modificació de la pàgina del producte per fix-serving-size-bot.
La pàgina del producte, també editada per fabi2, ficello, kiliweb, magasins-u, mairoluin, openfoodfacts-contributors, packbot, sebleouf, thaialagata, yuka.WjUwUlFaWU51S2tBcXNReW9FTDIyc3grOVliNVEycStPdUJLSVE9PQ.