Ajuda'ns a fer que la transparència alimentària sigui la norma!
Com a organització sense ànim de lucre, depenem de les vostres donacions per continuar informant els consumidors de tot el món sobre tot allò què mengen.
La revolució alimentària comença amb tu!
Petit Écolier Choco Pasión - LU - 150 g
Petit Écolier Choco Pasión - LU - 150 g
Aquesta pàgina del producte no està completa. Podeu ajudar a completar-la editant-la i afegint-hi més dades a partir de les fotos ja disponibles, o fent-ne més amb l'aplicació de androide o iPhone / iPad. Gràcies!
×
Codi de barres: 3017760311496 (EAN / EAN-13)
Quantitat: 150 g
Marques: LU
Categories: Snacks, Aperitius dolços, en:Specific products, Galetes i pastissos, en:Products for specific diets, Galetes, en:Products without gluten, Galetes de xocolata, en:Dark chocolate biscuits, en:Biscuit with a chocolate bar covering, en:Biscuit with a dark chocolate bar covering, en:Gluten-free biscuits
Països on es va vendre: Espanya
Matching with your preferences
Salut
Ingredients
-
20 ingredients
: Galleta (52 %): Harina de trigo (68 %), azúcar, mantequilla concentrada, leche desnatada en polvo, gasificantes (carbonato ácido de amonio, carbonato ácido de sodio, difosfato disódico), sal, regulador de acidez (ácido cítrico). Chocolate negro (48 %): Pasta de cacao, azúcar, manteca de cacao, grasa de leche anhidra, emulgente (lecitina de soja). (Cacao: 70 % mínimo). Puede contener frutos de cáscara.Al·lèrgens: en:Gluten, en:SoybeansRastres: en:Nuts
Processament d'aliments
-
Aliments ultra processats
Elements que indiquen que el producte està al grup 4 - Aliments i begudes ultraprocessats:
- Additiu: E322 - Lecitines
- Additiu: E450 - Difosfat
- Ingredient: Emulsionant
Els productes alimentaris es classifiquen en 4 grups segons el seu grau de processament:
- Aliments no processats o mínimament processats
- Ingredients culinaris processats
- Aliments processats
- Aliments ultra processats
La determinació del grup es fa en funció de la categoria del producte i dels ingredients que conté.
Additius
-
E322 - Lecitines
Lecithin: Lecithin -UK: , US: , from the Greek lekithos, "egg yolk"- is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues, which are amphiphilic – they attract both water and fatty substances -and so are both hydrophilic and lipophilic-, and are used for smoothing food textures, dissolving powders -emulsifying-, homogenizing liquid mixtures, and repelling sticking materials.Lecithins are mixtures of glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acid.Lecithin was first isolated in 1845 by the French chemist and pharmacist Theodore Gobley. In 1850, he named the phosphatidylcholine lécithine. Gobley originally isolated lecithin from egg yolk—λέκιθος lekithos is "egg yolk" in Ancient Greek—and established the complete chemical formula of phosphatidylcholine in 1874; in between, he had demonstrated the presence of lecithin in a variety of biological matters, including venous blood, in human lungs, bile, human brain tissue, fish eggs, fish roe, and chicken and sheep brain. Lecithin can easily be extracted chemically using solvents such as hexane, ethanol, acetone, petroleum ether, benzene, etc., or extraction can be done mechanically. It is usually available from sources such as soybeans, eggs, milk, marine sources, rapeseed, cottonseed, and sunflower. It has low solubility in water, but is an excellent emulsifier. In aqueous solution, its phospholipids can form either liposomes, bilayer sheets, micelles, or lamellar structures, depending on hydration and temperature. This results in a type of surfactant that usually is classified as amphipathic. Lecithin is sold as a food additive and dietary supplement. In cooking, it is sometimes used as an emulsifier and to prevent sticking, for example in nonstick cooking spray.Origen: Wikipedia (Anglès)
-
E322i - Lecitina
Lecithin: Lecithin -UK: , US: , from the Greek lekithos, "egg yolk"- is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues, which are amphiphilic – they attract both water and fatty substances -and so are both hydrophilic and lipophilic-, and are used for smoothing food textures, dissolving powders -emulsifying-, homogenizing liquid mixtures, and repelling sticking materials.Lecithins are mixtures of glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acid.Lecithin was first isolated in 1845 by the French chemist and pharmacist Theodore Gobley. In 1850, he named the phosphatidylcholine lécithine. Gobley originally isolated lecithin from egg yolk—λέκιθος lekithos is "egg yolk" in Ancient Greek—and established the complete chemical formula of phosphatidylcholine in 1874; in between, he had demonstrated the presence of lecithin in a variety of biological matters, including venous blood, in human lungs, bile, human brain tissue, fish eggs, fish roe, and chicken and sheep brain. Lecithin can easily be extracted chemically using solvents such as hexane, ethanol, acetone, petroleum ether, benzene, etc., or extraction can be done mechanically. It is usually available from sources such as soybeans, eggs, milk, marine sources, rapeseed, cottonseed, and sunflower. It has low solubility in water, but is an excellent emulsifier. In aqueous solution, its phospholipids can form either liposomes, bilayer sheets, micelles, or lamellar structures, depending on hydration and temperature. This results in a type of surfactant that usually is classified as amphipathic. Lecithin is sold as a food additive and dietary supplement. In cooking, it is sometimes used as an emulsifier and to prevent sticking, for example in nonstick cooking spray.Origen: Wikipedia (Anglès)
-
E330 - Acid citric
Citric acid: Citric acid is a weak organic acid that has the chemical formula C6H8O7. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than a million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring and chelating agent.A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate ion is written as C6H5O3−7 or C3H5O-COO-3−3.Origen: Wikipedia (Anglès)
-
E500 - Carbonats de sodi
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Origen: Wikipedia (Anglès)
-
E500ii - Bicarbonat de sodi
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Origen: Wikipedia (Anglès)
-
E503 - Carbonatos de amonio
Ammonium carbonate: Ammonium carbonate is a salt with the chemical formula -NH4-2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn.Origen: Wikipedia (Anglès)
-
E503ii - Carbonat àcid d'amoni
Ammonium carbonate: Ammonium carbonate is a salt with the chemical formula -NH4-2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn.Origen: Wikipedia (Anglès)
Anàlisi dels ingredients
-
Pot contenir oli de palma
Ingredients que poden contenir oli de palma: en:Butterfat
-
No és vegà
Ingredients no vegans: en:Butterfat, Llet desnatada en polsAlguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
-
Es desconeix si és vegetarià
Ingredients no reconeguts: Galetes, es:grasa-de-leche-anhidraAlguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
-
Detalls de l'anàlisi dels ingredients
Necessitem la teva ajuda!
Alguns ingredients no s'han pogut reconèixer.
Necessitem la teva ajuda!
Podeu ajudar-nos a reconèixer més ingredients i analitzar millor la llista d'ingredients d'aquest producte i d'altres mitjançant:
- Editeu aquesta pàgina de producte per corregir les faltes d’ortografia de la llista d’ingredients i/o per eliminar els ingredients d’altres idiomes i frases que no estiguin relacionades amb els ingredients.
- Afegiu entrades, sinònims o traduccions noves a les nostres llistes multilingües d’ingredients, mètodes de processament d’ingredients i etiquetes.
Uniu-vos al canal #ingredients del nostre espai de discussió a Slack i/o apreneu sobre l'anàlisi dels ingredients en la nostra wiki, si voleu ajudar. Gràcies!
: Galleta 52% (Harina de trigo 68%), azúcar, mantequilla concentrada, leche desnatada en polvo, gasificantes (carbonato ácido de amonio, carbonato ácido de sodio, difosfato disódico), sal, regulador de acidez (ácido cítrico), Chocolate negro 48% (Pasta de cacao), azúcar, manteca de cacao, grasa de leche anhidra, emulgente (lecitina de soja, Cacao 70%)- Galleta -> en:biscuit - ciqual_food_code: 24000 - percent: 52
- Harina de trigo -> en:wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410 - percent: 68
- azúcar -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016
- mantequilla concentrada -> en:butterfat - vegan: no - vegetarian: yes - from_palm_oil: maybe - ciqual_food_code: 16401
- leche desnatada en polvo -> en:skimmed-milk-powder - vegan: no - vegetarian: yes - ciqual_food_code: 19054
- gasificantes -> en:raising-agent
- carbonato ácido de amonio -> en:e503ii - vegan: yes - vegetarian: yes
- carbonato ácido de sodio -> en:e500ii - vegan: yes - vegetarian: yes
- difosfato disódico -> en:e450i - vegan: yes - vegetarian: yes
- sal -> en:salt - vegan: yes - vegetarian: yes - ciqual_food_code: 11058
- regulador de acidez -> en:acidity-regulator
- ácido cítrico -> en:e330 - vegan: yes - vegetarian: yes
- Chocolate negro -> en:dark-chocolate - vegan: maybe - vegetarian: yes - ciqual_proxy_food_code: 31074 - percent: 48
- Pasta de cacao -> en:cocoa-paste - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 16030
- azúcar -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016
- manteca de cacao -> en:cocoa-butter - vegan: yes - vegetarian: yes - ciqual_food_code: 16030
- grasa de leche anhidra -> es:grasa-de-leche-anhidra
- emulgente -> en:emulsifier
- lecitina de soja -> en:soya-lecithin - vegan: yes - vegetarian: yes - ciqual_food_code: 42200
- Cacao -> en:cocoa - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 18100 - percent: 70
Nutrició
-
Mala qualitat nutricional
⚠ ️Atenció: la quantitat de fibra no s'especifica, no es tindrà en compte la seva possible contribució positiva en la qualificació.⚠ ️Atenció: la quantitat de fruita, verdura i fruits secs no s'especifica a l'etiqueta, s'ha fet una estimació a partir de la llista d'ingredients: 0Aquest producte no es considera una beguda per al càlcul de la Nutri-Score.
Punts positius: 0
- Proteïnes: 4 / 5 (valor: 7.9, valor arrodonit: 7.9)
- Fibra: 0 / 5 (valor: 0, valor arrodonit: 0)
- Fruites, verdures, fruits secs i olis de colza/nou/oliva: 0 / 5 (valor: 0, valor arrodonit: 0)
Punts negatius: 23
- Energia: 6 / 10 (valor: 2138, valor arrodonit: 2138)
- Sucres: 5 / 10 (valor: 25, valor arrodonit: 25)
- Greixos saturats: 10 / 10 (valor: 18, valor arrodonit: 18)
- Sodi: 2 / 10 (valor: 224, valor arrodonit: 224)
Els punts per proteïnes no es compten perquè els punts negatius són més o iguals a 11.
Puntuació nutricional: (23 - 0)
Nutri-Score:
-
Nivells de nutrients
-
Greix en alta quantitat (29%)
Què us cal saber- Un alt consum de greixos, especialment de greixos saturats, pot augmentar el colesterol, que augmenta el risc de patir malalties del cor.
Recomanació: Reduïu el consum de greixos i greixos saturats- Trieu productes amb menys greixos i greixos saturats.
-
Àcid gras saturat en alta quantitat (18%)
Què us cal saber- Un alt consum de greixos, especialment de greixos saturats, pot augmentar el colesterol, que augmenta el risc de patir malalties del cor.
Recomanació: Reduïu el consum de greixos i greixos saturats- Trieu productes amb menys greixos i greixos saturats.
-
Sucre en alta quantitat (25%)
Què us cal saber- Un alt consum de sucre pot provocar augment de pes i càries dental. També augmenta el risc de patir diabetis tipus 2 i malalties cardiovasculars.
Recomanació: Limitau el consum de sucre i de begudes ensucrades- Les begudes ensucrades (com ara refrescos, begudes de fruites i sucs i nèctars de fruites) s'han de limitar tant com sigui possible (no més d'1 got al dia).
- Triau productes amb menor contingut de sucre i reduïu el consum de productes amb sucres afegits.
-
Sal comuna en Quantitat moderada (0.56%)
Què us cal saber- Un alt consum de sal (o sodi) pot provocar un augment de la pressió arterial, que pot augmentar el risc de patir malalties del cor i ictus.
- Moltes persones que tenen hipertensió no ho saben, ja que sovint no en tenen símptomes.
- La majoria de la gent consumeix massa sal (de 9 a 12 grams de mitjana al dia), al voltant del doble del nivell màxim d'ingesta recomanat.
Recomanació: Limitau la ingesta de sal i d'aliments rics en sal- Reduïu la sal que emprau quan cuinau, i no afegiu sal a taula.
- Limiteu el consum d'aperitius salats i trieu productes amb menor contingut de sal.
-
-
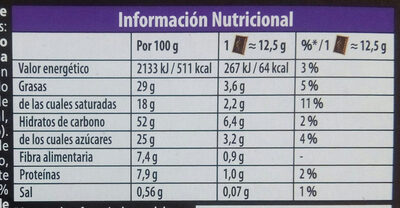
Informació nutricional
Informació nutricional Com es ven
per 100 g/100 mlComparat amb: en:Biscuit with a dark chocolate bar covering Energia 2.138 kj
(511 kcal)+3% Greix 29 g +24% Àcid gras saturat 18 g +28% Hidrats de carboni 52 g -18% Sucre 25 g -21% Fiber ? Proteïna 7,9 g +23% Sal comuna 0,56 g +14% Fruits‚ vegetables‚ nuts and rapeseed‚ walnut and olive oils (estimate from ingredients list analysis) 0 %
Entorn
-
Eco-puntuació D - Impacte ambiental alt
El Eco-Score és una puntuació experimental que resumeix els impactes ambientals dels productes alimentaris.→ L'Eco-Score es va desenvolupar inicialment a França i s'està ampliant per a altres països europeus. La fórmula Eco-Score està subjecta a canvis, ja que es millora periòdicament per fer-la més precisa i més adequada per a cada país.Anàlisi del cicle de vida
-
Impacte mitjà dels productes de la mateixa categoria: C (Score: 52/100)
Categoria: Biscuit (cookie), covering with a chocolate bar
Categoria: Biscuit (cookie), covering with a chocolate bar
- Puntuació ambiental PEF ( petjada ambiental de l'aliment ): 0.52 (com més baixa sigui la puntuació, menor serà l'impacte)
- incloent l'impacte sobre el canvi climàtic: 6.74 kg CO₂ eq/kg del producte
Etapa Impacte Agricultura
52.4 %Processament
41.6 %Empaquetament
2.1 %Transport
2.9 %Distribució
0.9 %Consum
0.0 %
Bonificacions i punts negatius
-
Falta informació sobre l'origen dels ingredients
Punts negatius: -5
⚠ ️ L'origen dels ingredients d'aquest producte no està indicat.
Si estan indicats a l'embalatge, podeu modificar la fitxa del producte i afegir-los.
Si sou el fabricant d'aquest producte, podeu enviar-nos la informació amb la nostra plataforma gratuïta per a productors.
-
Falta informació sobre l'embalatge d'aquest producte
Punts negatius: -15
⚠ ️ La informació sobre l'embalatge d'aquest producte no està completada.⚠ ️ Per a un càlcul més precís de l'Eco-Score, podeu modificar la pàgina del producte i afegir-los.
Si sou el fabricant d'aquest producte, podeu enviar-nos la informació amb la nostra plataforma gratuïta per a productors.
Eco-Score per a aquest producte
-
Impacte per a aquest producte: D (Score: 32/100)
Producte: Petit Écolier Choco Pasión - LU - 150 g
Puntuació de l'anàlisi del cicle de vida: 52
Suma de bonificacions i punts negatius: -20
Puntuació final: 32/100
-
Petjada de carboni
-
Equivalent a conduir 3.5 km en un cotxe de gasolina
674 g de CO² per cada 100 g de producte
La xifra d'emissions de carboni prové de la base de dades Agribalyse d'ADEME, per a la categoria: Biscuit (cookie), covering with a chocolate bar (Font: Base de dades ADEME Agribalyse)
Etapa Impacte Agricultura
36.3 %Processament
59.2 %Empaquetament
1.6 %Transport
2.6 %Distribució
0.3 %Consum
0.0 %
Empaquetament
-
Falta informació sobre l'embalatge d'aquest producte
⚠ ️ La informació sobre l'embalatge d'aquest producte no està completada.Take a photo of the recycling information Take a photo of the recycling information
Transport
-
Orígens dels ingredients
Falta informació sobre l'origen dels ingredients
⚠ ️ L'origen dels ingredients d'aquest producte no està indicat.
Si estan indicats a l'embalatge, podeu modificar la fitxa del producte i afegir-los.
Si sou el fabricant d'aquest producte, podeu enviar-nos la informació amb la nostra plataforma gratuïta per a productors.Add the origins of ingredients for this product Add the origins of ingredients for this product
Report a problem
-
Incomplete or incorrect information?
Category, labels, ingredients, allergens, nutritional information, photos etc.
If the information does not match the information on the packaging, please complete or correct it. Open Food Facts is a collaborative database, and every contribution is useful for all.
Fonts de dades
Producte afegit per kiliweb
Última modificació de la pàgina del producte per moon-rabbit.
La pàgina del producte, també editada per jbarcelona, musarana, openfoodfacts-contributors, yuka.U1Awd1RZWUNtOWN4bXZkZzRCRHY1SXdrLzdtWGMwYVpjZU1kSVE9PQ, yuka.WW9jYlMvUUxoZVkxbWZBWHAwaUsvZUpSNVozd2UyT3hlL05OSVE9PQ.