Ajuda'ns a fer que la transparència alimentària sigui la norma!
Com a organització sense ànim de lucre, depenem de les vostres donacions per continuar informant els consumidors de tot el món sobre tot allò què mengen.
La revolució alimentària comença amb tu!
KAFFEREP Raspberry - IKEA - 176g
KAFFEREP Raspberry - IKEA - 176g
Aquesta pàgina del producte no està completa. Podeu ajudar a completar-la editant-la i afegint-hi més dades a partir de les fotos ja disponibles, o fent-ne més amb l'aplicació de androide o iPhone / iPad. Gràcies!
×
Codi de barres: 1103749230000 (EAN / EAN-13)
Quantitat: 176g
Empaquetament: en:pp film-packet
Marques: IKEA
Categories: Snacks, Aperitius dolços, Galetes i pastissos, Galetes
Botigues: Ikea
Països on es va vendre: França, Alemanya, Espanya, Regne Unit
Matching with your preferences
Salut
Ingredients
-
27 ingredients
: Farine de blé, sucre, matières grasses végétales (palme, noix de coco), sirop de glucose, humectant (E422), sirop de glucose fructose, lait écrémé en poudre, poudres à lever (E450, E500, E503), jus de framboise concentré (0,4 %), sel, amidon de blé, émulsifiant (lécithine de tournesol), stabilisant (E407), jus de myrtille concentré, jus de sureau concentré, correcteurs d'acidité (E330, E331, E270), arômes naturels.Al·lèrgens: en:GlutenRastres: en:Eggs, en:Nuts
Processament d'aliments
-
Aliments ultra processats
Elements que indiquen que el producte està al grup 4 - Aliments i begudes ultraprocessats:
- Additiu: E322 - Lecitines
- Additiu: E407 - Carragahen
- Additiu: E422 - Glicerol
- Additiu: E450 - Difosfat
- Ingredient: Emulsionant
- Ingredient: Aromes
- Ingredient: Glucosa
- Ingredient: Xarop de glucosa
- Ingredient: Humectant
Els productes alimentaris es classifiquen en 4 grups segons el seu grau de processament:
- Aliments no processats o mínimament processats
- Ingredients culinaris processats
- Aliments processats
- Aliments ultra processats
La determinació del grup es fa en funció de la categoria del producte i dels ingredients que conté.
Additius
-
E270 - Àcid làctic
Lactic acid: Lactic acid is an organic compound with the formula CH3CH-OH-COOH. In its solid state, it is white and water-soluble. In its liquid state, it is colorless. It is produced both naturally and synthetically. With a hydroxyl group adjacent to the carboxyl group, lactic acid is classified as an alpha-hydroxy acid -AHA-. In the form of its conjugate base called lactate, it plays a role in several biochemical processes. In solution, it can ionize a proton from the carboxyl group, producing the lactate ion CH3CH-OH-CO−2. Compared to acetic acid, its pKa is 1 unit less, meaning lactic acid deprotonates ten times more easily than acetic acid does. This higher acidity is the consequence of the intramolecular hydrogen bonding between the α-hydroxyl and the carboxylate group. Lactic acid is chiral, consisting of two optical isomers. One is known as L--+--lactic acid or -S--lactic acid and the other, its mirror image, is D--−--lactic acid or -R--lactic acid. A mixture of the two in equal amounts is called DL-lactic acid, or racemic lactic acid. Lactic acid is hygroscopic. DL-lactic acid is miscible with water and with ethanol above its melting point which is around 17 or 18 °C. D-lactic acid and L-lactic acid have a higher melting point. In animals, L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase -LDH- in a process of fermentation during normal metabolism and exercise. It does not increase in concentration until the rate of lactate production exceeds the rate of lactate removal, which is governed by a number of factors, including monocarboxylate transporters, concentration and isoform of LDH, and oxidative capacity of tissues. The concentration of blood lactate is usually 1–2 mM at rest, but can rise to over 20 mM during intense exertion and as high as 25 mM afterward. In addition to other biological roles, L-lactic acid is the primary endogenous agonist of hydroxycarboxylic acid receptor 1 -HCA1-, which is a Gi/o-coupled G protein-coupled receptor -GPCR-.In industry, lactic acid fermentation is performed by lactic acid bacteria, which convert simple carbohydrates such as glucose, sucrose, or galactose to lactic acid. These bacteria can also grow in the mouth; the acid they produce is responsible for the tooth decay known as caries. In medicine, lactate is one of the main components of lactated Ringer's solution and Hartmann's solution. These intravenous fluids consist of sodium and potassium cations along with lactate and chloride anions in solution with distilled water, generally in concentrations isotonic with human blood. It is most commonly used for fluid resuscitation after blood loss due to trauma, surgery, or burns.Origen: Wikipedia (Anglès)
-
E322 - Lecitines
Lecithin: Lecithin -UK: , US: , from the Greek lekithos, "egg yolk"- is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues, which are amphiphilic – they attract both water and fatty substances -and so are both hydrophilic and lipophilic-, and are used for smoothing food textures, dissolving powders -emulsifying-, homogenizing liquid mixtures, and repelling sticking materials.Lecithins are mixtures of glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acid.Lecithin was first isolated in 1845 by the French chemist and pharmacist Theodore Gobley. In 1850, he named the phosphatidylcholine lécithine. Gobley originally isolated lecithin from egg yolk—λέκιθος lekithos is "egg yolk" in Ancient Greek—and established the complete chemical formula of phosphatidylcholine in 1874; in between, he had demonstrated the presence of lecithin in a variety of biological matters, including venous blood, in human lungs, bile, human brain tissue, fish eggs, fish roe, and chicken and sheep brain. Lecithin can easily be extracted chemically using solvents such as hexane, ethanol, acetone, petroleum ether, benzene, etc., or extraction can be done mechanically. It is usually available from sources such as soybeans, eggs, milk, marine sources, rapeseed, cottonseed, and sunflower. It has low solubility in water, but is an excellent emulsifier. In aqueous solution, its phospholipids can form either liposomes, bilayer sheets, micelles, or lamellar structures, depending on hydration and temperature. This results in a type of surfactant that usually is classified as amphipathic. Lecithin is sold as a food additive and dietary supplement. In cooking, it is sometimes used as an emulsifier and to prevent sticking, for example in nonstick cooking spray.Origen: Wikipedia (Anglès)
-
E322i - Lecitina
Lecithin: Lecithin -UK: , US: , from the Greek lekithos, "egg yolk"- is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues, which are amphiphilic – they attract both water and fatty substances -and so are both hydrophilic and lipophilic-, and are used for smoothing food textures, dissolving powders -emulsifying-, homogenizing liquid mixtures, and repelling sticking materials.Lecithins are mixtures of glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acid.Lecithin was first isolated in 1845 by the French chemist and pharmacist Theodore Gobley. In 1850, he named the phosphatidylcholine lécithine. Gobley originally isolated lecithin from egg yolk—λέκιθος lekithos is "egg yolk" in Ancient Greek—and established the complete chemical formula of phosphatidylcholine in 1874; in between, he had demonstrated the presence of lecithin in a variety of biological matters, including venous blood, in human lungs, bile, human brain tissue, fish eggs, fish roe, and chicken and sheep brain. Lecithin can easily be extracted chemically using solvents such as hexane, ethanol, acetone, petroleum ether, benzene, etc., or extraction can be done mechanically. It is usually available from sources such as soybeans, eggs, milk, marine sources, rapeseed, cottonseed, and sunflower. It has low solubility in water, but is an excellent emulsifier. In aqueous solution, its phospholipids can form either liposomes, bilayer sheets, micelles, or lamellar structures, depending on hydration and temperature. This results in a type of surfactant that usually is classified as amphipathic. Lecithin is sold as a food additive and dietary supplement. In cooking, it is sometimes used as an emulsifier and to prevent sticking, for example in nonstick cooking spray.Origen: Wikipedia (Anglès)
-
E330 - Acid citric
Citric acid: Citric acid is a weak organic acid that has the chemical formula C6H8O7. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than a million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring and chelating agent.A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate ion is written as C6H5O3−7 or C3H5O-COO-3−3.Origen: Wikipedia (Anglès)
-
E331 - Citrats de sodi
Sodium citrate: Sodium citrate may refer to any of the sodium salts of citrate -though most commonly the third-: Monosodium citrate Disodium citrate Trisodium citrateThe three forms of the salt are collectively known by the E number E331. Sodium citrates are used as acidity regulators in food and drinks, and also as emulsifiers for oils. They enable cheeses to melt without becoming greasy.Origen: Wikipedia (Anglès)
-
E407 - Carragahen
Carrageenan: Carrageenans or carrageenins - karr-ə-gee-nənz, from Irish carraigín, "little rock"- are a family of linear sulfated polysaccharides that are extracted from red edible seaweeds. They are widely used in the food industry, for their gelling, thickening, and stabilizing properties. Their main application is in dairy and meat products, due to their strong binding to food proteins. There are three main varieties of carrageenan, which differ in their degree of sulfation. Kappa-carrageenan has one sulfate group per disaccharide, iota-carrageenan has two, and lambda-carrageenan has three. Gelatinous extracts of the Chondrus crispus -Irish moss- seaweed have been used as food additives since approximately the fifteenth century. Carrageenan is a vegetarian and vegan alternative to gelatin in some applications or may be used to replace gelatin in confectionery.Origen: Wikipedia (Anglès)
-
E422 - Glicerol
Glycerol: Glycerol -; also called glycerine or glycerin; see spelling differences- is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in all lipids known as triglycerides. It is widely used in the food industry as a sweetener and humectant and in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature.Origen: Wikipedia (Anglès)
-
E500 - Carbonats de sodi
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Origen: Wikipedia (Anglès)
-
E503 - Carbonatos de amonio
Ammonium carbonate: Ammonium carbonate is a salt with the chemical formula -NH4-2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn.Origen: Wikipedia (Anglès)
Anàlisi dels ingredients
-
Oli de palma
Ingredients que contenen oli de palma: Greix de palma
-
No és vegà
Ingredients no vegans: Llet desnatada en pols
-
Pot ser vegetarià
Ingredients que potser no són vegetarians: E422, Aroma natural
-
Detalls de l'anàlisi dels ingredients
: Farine de blé, sucre, matières grasses végétales de palme, matières grasses végétales de noix de coco, sirop de glucose, humectant (e422), sirop de glucose fructose, lait écrémé en poudre, poudres à lever (e450, e500, e503), jus de framboise concentré 0.4%, sel, amidon de blé, émulsifiant (lécithine de tournesol), stabilisant (e407), myrtille, jus de sureau concentré, correcteurs d'acidité (e330, e331, e270), arômes naturels- Farine de blé -> en:wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410 - percent_min: 5.55555555555556 - percent_max: 96.4
- sucre -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 0.4 - percent_max: 37
- matières grasses végétales de palme -> en:palm-fat - vegan: yes - vegetarian: yes - from_palm_oil: yes - ciqual_proxy_food_code: 16129 - percent_min: 0.4 - percent_max: 32.4
- matières grasses végétales de noix de coco -> en:coconut-oil - vegan: yes - vegetarian: yes - from_palm_oil: no - ciqual_food_code: 16040 - percent_min: 0.4 - percent_max: 24.4
- sirop de glucose -> en:glucose-syrup - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 0.4 - percent_max: 19.6
- humectant -> en:humectant - percent_min: 0.4 - percent_max: 16.4
- e422 -> en:e422 - vegan: maybe - vegetarian: maybe - percent_min: 0.4 - percent_max: 16.4
- sirop de glucose fructose -> en:glucose-fructose-syrup - vegan: yes - vegetarian: yes - ciqual_food_code: 31077 - percent_min: 0.4 - percent_max: 14.1142857142857
- lait écrémé en poudre -> en:skimmed-milk-powder - vegan: no - vegetarian: yes - ciqual_food_code: 19054 - percent_min: 0.4 - percent_max: 12.4
- poudres à lever -> en:raising-agent - percent_min: 0.4 - percent_max: 11.0666666666667
- e450 -> en:e450 - vegan: yes - vegetarian: yes - percent_min: 0.133333333333333 - percent_max: 11.0666666666667
- e500 -> en:e500 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 5.53333333333333
- e503 -> en:e503 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 3.68888888888889
- jus de framboise concentré -> en:concentrated-raspberry-juice - vegan: yes - vegetarian: yes - ciqual_food_code: 13015 - percent_min: 0.4 - percent: 0.4 - percent_max: 0.4
- sel -> en:salt - vegan: yes - vegetarian: yes - ciqual_food_code: 11058 - percent_min: 0 - percent_max: 0.4
- amidon de blé -> en:wheat-starch - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9510 - percent_min: 0 - percent_max: 0.4
- émulsifiant -> en:emulsifier - percent_min: 0 - percent_max: 0.4
- lécithine de tournesol -> en:sunflower-lecithin - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 0.4
- stabilisant -> en:stabiliser - percent_min: 0 - percent_max: 0.4
- e407 -> en:e407 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 0.4
- myrtille -> en:blueberry - vegan: yes - vegetarian: yes - ciqual_food_code: 13028 - percent_min: 0 - percent_max: 0.4
- jus de sureau concentré -> en:elderberry-juice-concentrate - vegan: yes - vegetarian: yes - ciqual_food_code: 13126 - percent_min: 0 - percent_max: 0.4
- correcteurs d'acidité -> en:acidity-regulator - percent_min: 0 - percent_max: 0.4
- e330 -> en:e330 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 0.4
- e331 -> en:e331 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 0.2
- e270 -> en:e270 - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 0.133333333333333
- arômes naturels -> en:natural-flavouring - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 0.4
Nutrició
-
Mala qualitat nutricional
⚠ ️Atenció: la quantitat de fibra no s'especifica, no es tindrà en compte la seva possible contribució positiva en la qualificació.⚠ ️Atenció: la quantitat de fruita, verdura i fruits secs no s'especifica a l'etiqueta, s'ha fet una estimació a partir de la llista d'ingredients: 0Aquest producte no es considera una beguda per al càlcul de la Nutri-Score.
Punts positius: 0
- Proteïnes: 2 / 5 (valor: 4.2, valor arrodonit: 4.2)
- Fibra: 0 / 5 (valor: 0, valor arrodonit: 0)
- Fruites, verdures, fruits secs i olis de colza/nou/oliva: 0 / 5 (valor: 0.4, valor arrodonit: 0.4)
Punts negatius: 25
- Energia: 5 / 10 (valor: 1966, valor arrodonit: 1966)
- Sucres: 8 / 10 (valor: 37, valor arrodonit: 37)
- Greixos saturats: 10 / 10 (valor: 12, valor arrodonit: 12)
- Sodi: 2 / 10 (valor: 210, valor arrodonit: 210)
Els punts per proteïnes no es compten perquè els punts negatius són més o iguals a 11.
Puntuació nutricional: (25 - 0)
Nutri-Score:
-
Nivells de nutrients
-
Greix en Quantitat moderada (19%)
Què us cal saber- Un alt consum de greixos, especialment de greixos saturats, pot augmentar el colesterol, que augmenta el risc de patir malalties del cor.
Recomanació: Reduïu el consum de greixos i greixos saturats- Trieu productes amb menys greixos i greixos saturats.
-
Àcid gras saturat en alta quantitat (12%)
Què us cal saber- Un alt consum de greixos, especialment de greixos saturats, pot augmentar el colesterol, que augmenta el risc de patir malalties del cor.
Recomanació: Reduïu el consum de greixos i greixos saturats- Trieu productes amb menys greixos i greixos saturats.
-
Sucre en alta quantitat (37%)
Què us cal saber- Un alt consum de sucre pot provocar augment de pes i càries dental. També augmenta el risc de patir diabetis tipus 2 i malalties cardiovasculars.
Recomanació: Limitau el consum de sucre i de begudes ensucrades- Les begudes ensucrades (com ara refrescos, begudes de fruites i sucs i nèctars de fruites) s'han de limitar tant com sigui possible (no més d'1 got al dia).
- Triau productes amb menor contingut de sucre i reduïu el consum de productes amb sucres afegits.
-
Sal comuna en Quantitat moderada (0.525%)
Què us cal saber- Un alt consum de sal (o sodi) pot provocar un augment de la pressió arterial, que pot augmentar el risc de patir malalties del cor i ictus.
- Moltes persones que tenen hipertensió no ho saben, ja que sovint no en tenen símptomes.
- La majoria de la gent consumeix massa sal (de 9 a 12 grams de mitjana al dia), al voltant del doble del nivell màxim d'ingesta recomanat.
Recomanació: Limitau la ingesta de sal i d'aliments rics en sal- Reduïu la sal que emprau quan cuinau, i no afegiu sal a taula.
- Limiteu el consum d'aperitius salats i trieu productes amb menor contingut de sal.
-
-
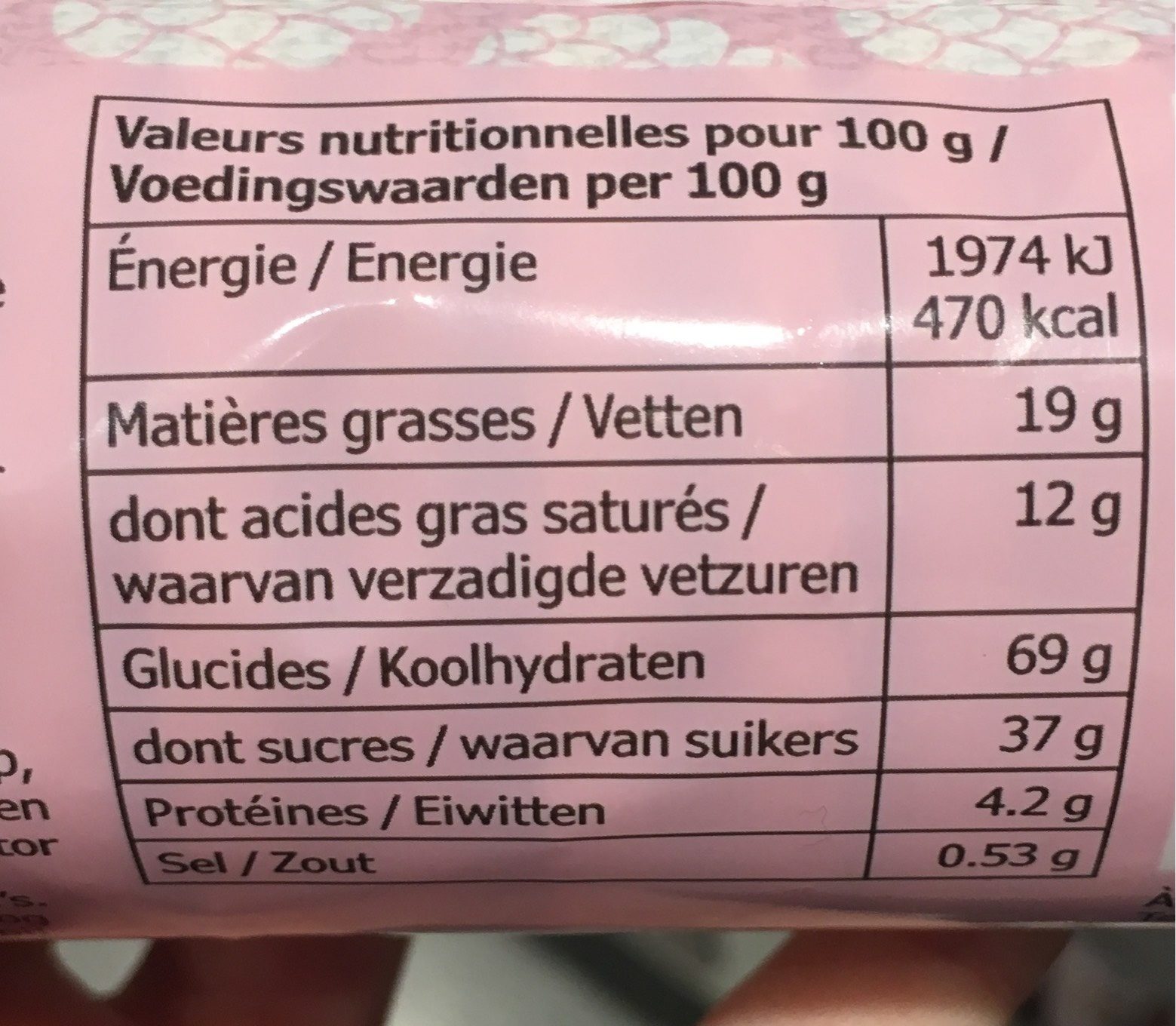
Informació nutricional
Informació nutricional Com es ven
per 100 g/100 mlComparat amb: Galetes Energia 1.966 kj
(470 kcal)-0% Greix 19 g -5% Àcid gras saturat 12 g +47% Hidrats de carboni 69 g +7% Sucre 37 g +50% Fiber ? Proteïna 4,2 g -35% Sal comuna 0,525 g -12% Fruits‚ vegetables‚ nuts and rapeseed‚ walnut and olive oils (estimate from ingredients list analysis) 0,4 %
Entorn
-
Eco-puntuació D - Impacte ambiental alt
El Eco-Score és una puntuació experimental que resumeix els impactes ambientals dels productes alimentaris.→ L'Eco-Score es va desenvolupar inicialment a França i s'està ampliant per a altres països europeus. La fórmula Eco-Score està subjecta a canvis, ja que es millora periòdicament per fer-la més precisa i més adequada per a cada país.Anàlisi del cicle de vida
-
Impacte mitjà dels productes de la mateixa categoria: B (Score: 69/100)
Categoria: Biscuit (cookie)
Categoria: Biscuit (cookie)
- Puntuació ambiental PEF ( petjada ambiental de l'aliment ): 0.35 (com més baixa sigui la puntuació, menor serà l'impacte)
- incloent l'impacte sobre el canvi climàtic: 2.88 kg CO₂ eq/kg del producte
Etapa Impacte Agricultura
80.5 %Processament
11.8 %Empaquetament
3.1 %Transport
3.2 %Distribució
1.4 %Consum
0.0 %
Bonificacions i punts negatius
-
Falta informació sobre l'origen dels ingredients
Punts negatius: -5
⚠ ️ L'origen dels ingredients d'aquest producte no està indicat.
Si estan indicats a l'embalatge, podeu modificar la fitxa del producte i afegir-los.
Si sou el fabricant d'aquest producte, podeu enviar-nos la informació amb la nostra plataforma gratuïta per a productors.
-
Ingredients que amenacen les espècies
Punts negatius: -10
Conté oli de palma
Els boscos tropicals d'Àsia, Àfrica i Amèrica Llatina es destrueixen per crear i ampliar les plantacions de palmera d'oli. La desforestació contribueix al canvi climàtic, i posa en perill espècies com l'orangutan, l'elefant pigmeu i el rinoceront de Sumatra.
-
Embalatge d'alt impacte
Punts negatius: -15
Forma Material Reciclatge Impacte Paquet PP 5 - polipropilè Alt Paquet PP 5 - polipropilè Alt
Eco-Score per a aquest producte
-
Impacte per a aquest producte: D (Score: 39/100)
Producte: KAFFEREP Raspberry - IKEA - 176g
Puntuació de l'anàlisi del cicle de vida: 69
Suma de bonificacions i punts negatius: -30
Puntuació final: 39/100
-
Petjada de carboni
-
Equivalent a conduir 1.5 km en un cotxe de gasolina
288 g de CO² per cada 100 g de producte
La xifra d'emissions de carboni prové de la base de dades Agribalyse d'ADEME, per a la categoria: Biscuit (cookie) (Font: Base de dades ADEME Agribalyse)
Etapa Impacte Agricultura
82.9 %Processament
7.9 %Empaquetament
3.8 %Transport
4.7 %Distribució
0.7 %Consum
0.0 %
Empaquetament
-
Embalatge d'alt impacte
-
Peces d'embalatge
Paquet (PP 5 - polipropilè)
Paquet (PP 5 - polipropilè)
-
Materials d'embalatge
Material % Pes de l'embalatge Pes de l'embalatge per 100 g de producte Plàstic
-
Transport
-
Orígens dels ingredients
Falta informació sobre l'origen dels ingredients
⚠ ️ L'origen dels ingredients d'aquest producte no està indicat.
Si estan indicats a l'embalatge, podeu modificar la fitxa del producte i afegir-los.
Si sou el fabricant d'aquest producte, podeu enviar-nos la informació amb la nostra plataforma gratuïta per a productors.Add the origins of ingredients for this product Add the origins of ingredients for this product
Espècies amenaçades
-
Conté oli de palma
Fomenta la desforestació i amenaça espècies com l'orangutan
Els boscos tropicals d'Àsia, Àfrica i Amèrica Llatina es destrueixen per crear i ampliar les plantacions de palmera d'oli. La desforestació contribueix al canvi climàtic, i posa en perill espècies com l'orangutan, l'elefant pigmeu i el rinoceront de Sumatra.
Report a problem
-
Incomplete or incorrect information?
Category, labels, ingredients, allergens, nutritional information, photos etc.
If the information does not match the information on the packaging, please complete or correct it. Open Food Facts is a collaborative database, and every contribution is useful for all.
Fonts de dades
Producte afegit per kiliweb
Última modificació de la pàgina del producte per foodvisor.
La pàgina del producte, també editada per acuario, ecoscore-impact-estimator, elcoco, foodless, jim-callan, openfoodfacts-contributors, quechoisir, roboto-app, swipe-studio, yuka.Eot5AtmmLtwhN8fv06w2_TaYMsC7XNMHIVQiog, yuka.SHAxY1NvUmVvL2tNa3R0aTd6N0g2OEJYL0ptR1lEdWFldUZNSUE9PQ, yuka.sY2b0xO6T85zoF3NwEKvlk4aSYGGqTvOKB7kqB2C2_CTKKTZcO510NDTNqg.